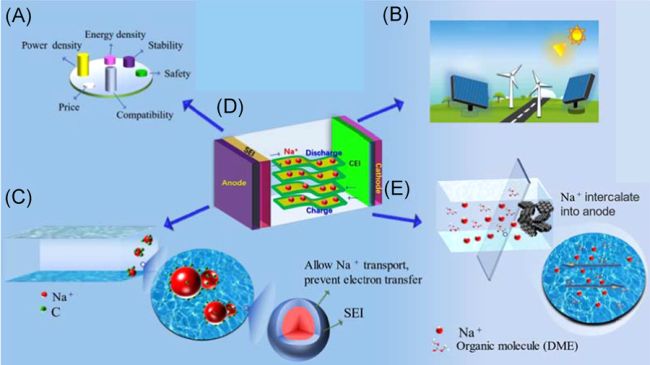
It’s important to find the alternative technologies for replacing the lithium-ion based energy storages as the awareness of protecting Earth’s precious resources grows and promise for sustainable development of human society strengthens. Sodium-ion based technologies for energy storage has emerged as a promising candidate to substitute lithium since 1985 when Na/S battery was invented. Even though SIB technology has gone a relatively long way, it’s still in its infancy but demonstrates all the viable aspects for the next generation of energy storage to go for. Sodium element is abundant and distributes widely on Earth, which potentially can greatly reduce the cost for mining and purifying compared to that for lithium. It’s been estimated that the price for sodium-ion based cells can be 20% to 40% lower than its counterpart lithium-ion cells. The market for the promising SIBs is getting readier and readier as reports show that the batteries for EV (Electric Vehicle) sector will increase from 0.6 TWh in 2022 to 2.8 TWh by 2030. In the base-case scenario, Sodium-ion batteries are seeking to take some share of LFP (Lithium Iron Phosphate) batteries in their dominating market of passenger EVs and energy storage applications and it’s estimated to reach 20 GWh by 2030.
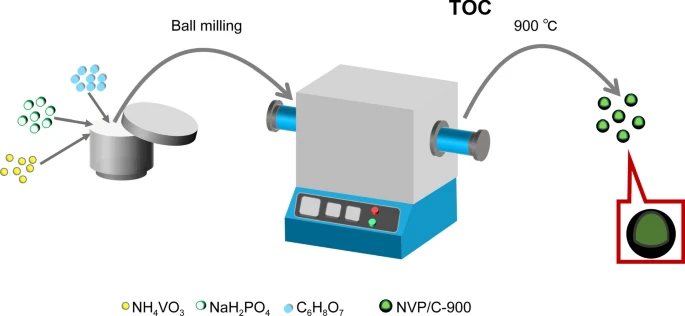
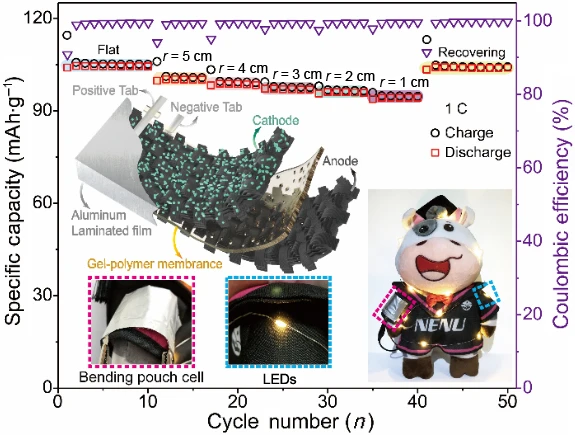
Like LIBs (Lithium-ion Batteries), SIBs have a cathode with sodium insertion materials and an anode. The cathode and anode are structurally separated by the electrolyte. There have been different SIBs assembled to meet the requirements for performance. When researchers cheer for the advantages of SIBs, such as high energy density, ease of fabrication and improved safety, they are also baffled by the rapid capacity fading of SIBs. Researchers have reported various solutions for improving the cycle life of SIBs. D. Wang, et. al., reported that the SIBs using carbon-coated sodium vanadium phosphate as cathode material can reach retention of 92.83 after 100 cycles and 47.92% after 3000 cycles, which is comparable to the LIBs. Q. Deng et. al. reported they achieved a 75.5% capacity retention and broad temperature adaptability by using 3D porous Fluorine-doped NaTi2(PO4)3@C as anode material. CD. Zhao et. al. reported their proposal for making flexible SIBs called QSFB (Quasi-Solid-State Sodium-Ion Full Battery) that can used in wearable applications.
One of the mechanisms behind the degradation of cycling stability has recently been discovered by a team of Cornell researchers led by Andrej Singer, an assistant professor of materials science and engineering. Andrej Singer’s team discovered the secret of a critical mechanism behind the degrading cycle rate of SIBs. When sodium ions move through the battery due to potential difference, multiple P2-O2 phase transitions in the P2-type layered cathode can happen as the layered oxides are charged and discharged by sodium ion intercalation and deintercalation. The indication of the occurrence of such a flawed phase transition is the sudden realignment of the layered structure from a disordered state. The phase transition was considered as irreversible and resulting in degraded cycle life of the SIBs. The team explained that the atoms realign and help trigger the undesirable phase transition during battery charge.
Thanks to the new X-ray imaging technique developed by the team using the Cornell High Energy Synchrotron Source, they were able to observe the unexpected atomic alignment within their battery sample. The findings have led to solutions to the degradation of cycle life of SIBs. One solution is to modify the battery chemistry to introduce a strategic disorder to the particles just before the flawed transition phase, according to Huang, a doctoral student and lead author of the report. “By changing the ratios of our transition metals, in this case, nickel and manganese,” Huang said, “we can introduce a bit of disorder and potentially reduce the ordering effect we observed.”
Reference
The weekend read: Sodium-ion batteries go mainstream – pv magazine International (pv-magazine.com)
Engineers reveal cause of key sodium-ion battery flaw | Cornell Chronicle
Huang, J. J., Weinstock, D., Hirsh, H., Bouck, R., Zhang, M., Gorobtsov, O. Y., Okamura, M., Harder, R., Cha, W., Ruff, J. P. C., Meng, Y. S., Singer, A., Disorder Dynamics in Battery Nanoparticles During Phase Transitions Revealed by Operando Single-Particle Diffraction. Adv. Energy Mater. 2022, 12, 2103521. https://doi.org/10.1002/aenm.202103521
Wang, D., Cai, P., Zou, GQ. et al. Ultra-stable carbon-coated sodium vanadium phosphate as cathode material for sodium-ion battery. Rare Met. 41, 115–124 (2022). https://doi.org/10.1007/s12598-021-01743-y
Qiang Deng, Qian Cheng, Xiaozhao Liu, Changdong Chen, Qianhui Huang, Jing Li, Wentao Zhong, Yijuan Li, Junhua Hu, Hua Wang, Lijue Wu, Chenghao Yang, 3D porous Fluorine-Doped NaTi2(PO4)3@C as High-Performance Sodium-Ion battery anode with broad temperature adaptability, Chemical Engineering Journal, Volume 430, Part 2, 2022, 132710, ISSN 1385-8947, https://doi.org/10.1016/j.cej.2021.132710
Zhao, CD., Guo, JZ., Gu, ZY. et al. Flexible quasi-solid-state sodium-ion full battery with ultralong cycle life, high energy density and high-rate capability. Nano Res. 15, 925–932 (2022). https://doi.org/10.1007/s12274-021-3577-7