Batteries are considered as one of the most important parts of mobile electronics. This situation will surely continue in near future before any self-powering designs can break through all the technological barriers and become practical in both applications and manufacturing. The mostly used batteries by mobile electronic devices are lithium ion-based batteries. From smart phones, handheld devices, vehicles, laptop, and security devices to IoT (Internet of Things) devices all over the world, the usage of lithium-ion batteries has been increasing from year to year.
Most of the lithium-ion batteries have liquid electrolytes that is made of lithium salt dissolved in an organic solvent, which transport the lithium ions between the battery’s cathode and anode to create current flow in the circuit. There are many advantages of liquid electrolytes-based lithium-ion batteries, but they have disadvantages that can be very serious. When high current is delivered, dendrites, small filaments of lithium metal can form in the liquid and cause short circuits in the battery. Also, the liquid solvent is flammable and toxic, and it can easily catch fire without protection and ventilation.
From the advent of lithium-ion batteries, researchers have studied how to improve the power density of the batteries while keep them operating safely. The conventional lithium-ion batteries with liquid electrolytes have much lower energy density. Therefore, it needs more batteries to achieve the same level of power output, which take up much more space in the device. The goal is to substitute the liquid electrolytes with solid-state electrolytes (SSE), which can prevent lithium metal penetration and can be made of materials that can not catch fire. Currently, most of the solid electrolytes are derived from ceramic materials. These ceramic materials are good conductors, but they are also bulky and brittle. Any small defects or uneven stresses induced during manufacturing and the process of charging or discharging can cause them to crack and break.
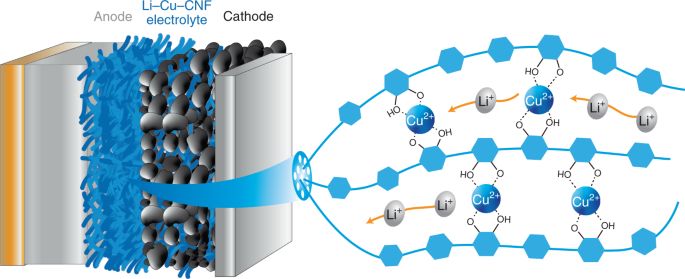
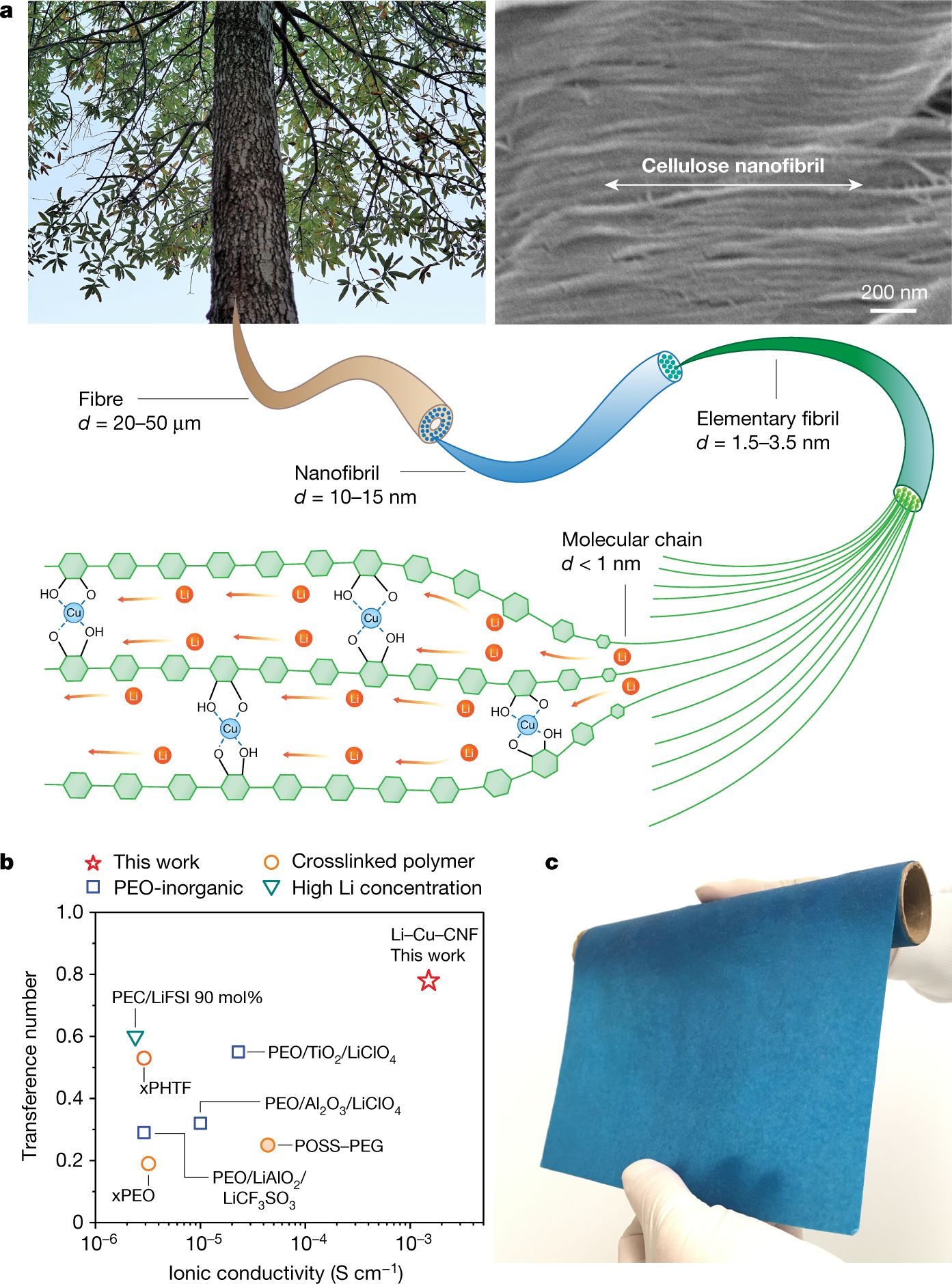
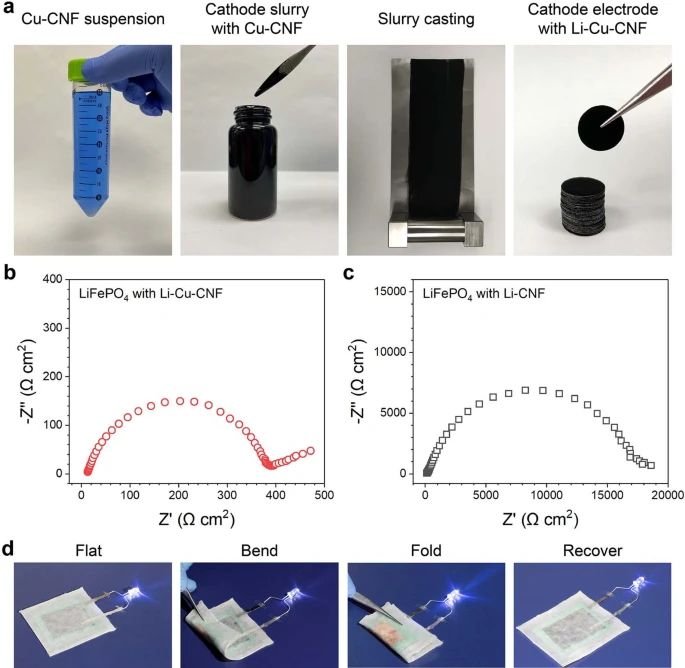
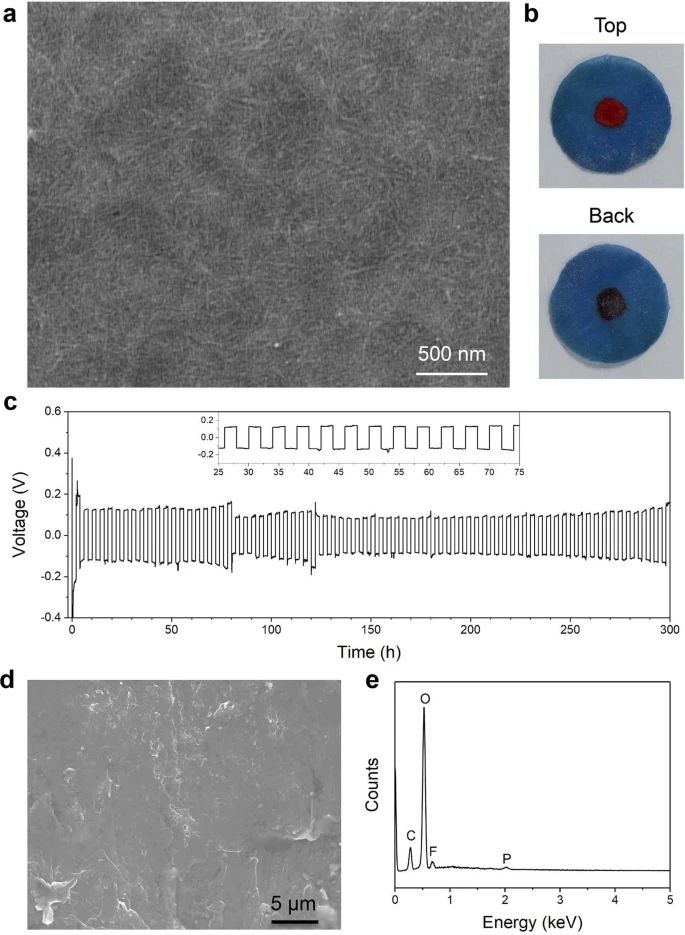
Some researchers have found alternative materials that can be excellent solid electrolyte. The studies conducted by a group of scientists from the University of Maryland and Brown University report that the new solid-state electrolyte is thin and flexible as a piece of paper, and it has comparable conductivity of the ceramic electrolytes. The new solid material for ion conducting is made of copper-coordinated cellulose nanofibrils, polymer tubes derived from wood. According to the paper that has been published in journal Nature, the conductivity for ion of the new solid electrolyte is 10 to 100 times better than other polymer ion conductors. The new material can be not only a solid electrolyte, but also as an ion-conducting binder for the cathode of an all-solid-state battery. A team led by Xin Li, associate professor of materials science at the Harvard John A. Paulson School of Engineering and Applied Science (SEAS) published their research on Nature May 12, 2021. Li and his team reported an innovative design of solid-state batteries based on a stable lithium-metal material that can be charged and discharged at least 10,000 times, far more than conventional lithium-ion batteries with maximum 300 to 500 charge cycles.

Reference
- https://news.harvard.edu/gazette/story/2021/05/researchers-design-long-lasting-solid-state-lithium-battery/
- Ye, L., Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021). https://doi.org/10.1038/s41586-021-03486-3
- Yang, C., Wu, Q., Xie, W. et al. Copper-coordinated cellulose ion conductors for solid-state batteries. Nature 598, 590–596 (2021). https://doi.org/10.1038/s41586-021-03885-6
- Lee, SY. Expanding cellulose. Nat Energy 6, 949–950 (2021). https://doi.org/10.1038/s41560-021-00907-5
- https://mse.umd.edu/news/story/expanded-wood-fiber-for-highperformance-solidstate-paper-batteries
- Eric Jianfeng Cheng, Takeshi Kimura, Mao Shoji, Hiroshi Ueda, Hirokazu Munakata, and Kiyoshi Kanamura, Ceramic-Based Flexible Sheet Electrolyte for Li Batteries, ACS Applied Materials & Interfaces 2020 12 (9), 10382-10388, DOI: 10.1021/acsami.9b21251
- https://spectrum.ieee.org/ion-storage-systems-ceramic-electrolyte-news-solid-state-batteries
- Takada, SECONDARY BATTERIES – LITHIUM RECHARGEABLE SYSTEMS – LITHIUM-ION | Electrolytes: Solid Oxide, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2013
- Xingwen Yu, Arumugam Manthiram, A review of composite polymer-ceramic electrolytes for lithium batteries, Energy Storage Materials, Volume 34, 2021, Pages 282-300, ISSN 2405-8297
- Masashi Kotobuki (NUS, Singapore), Shufeng Song (Chongqing University, China), Chao Chen (NUS, Singapore) and Li Lu (NUS, Singapore), Ceramic Electrolytes for All-Solid-State Li Batteries, https://doi.org/10.1142/10815 | July 2018
- Porz, L., Knez, D., Scherer, M. et al. Dislocations in ceramic electrolytes for solid-state Li batteries. Sci Rep 11, 8949 (2021). https://doi.org/10.1038/s41598-021-88370-w
- Lee, YG., Fujiki, S., Jung, C. et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat Energy 5, 299–308 (2020). https://doi.org/10.1038/s41560-020-0575-z
- Randau, S., Weber, D.A., Kötz, O. et al. Benchmarking the performance of all-solid-state lithium batteries. Nat Energy 5, 259–270 (2020). https://doi.org/10.1038/s41560-020-0565-1
- Chunwen Sun, Jin Liu, Yudong Gong, David P. Wilkinson, Jiujun Zhang, Recent advances in all-solid-state rechargeable lithium batteries, Nano Energy, Volume 33, 2017, Pages 363-386, ISSN 2211-2855
- Guanglei Cui, Reasonable Design of High-Energy-Density Solid-State Lithium-Metal Batteries, Matter, Volume 2, Issue 4, 2020, Pages 805-815, ISSN 2590-2385, https://doi.org/10.1016/j.matt.2020.02.003



